Research Article - (2019) Volume 9, Issue 2
Cancer new drug development and testing mostly rely on existing drug which plays a great role as templates, and the biomarkers. Thymidylate Synthase Inhibitors (TSI) tabulated below are available for the diseases conditions like cancer which encircle the thymidylate synthase (TS). Sparse data are available for TS involvement in cancer. TS is prudent in the synthesis of thymidylate which is useful for body functionalities, and its production pathways are observed to contain supportive proteins. The loss of thymine in cancerous cells couple in abnormal DNA synthesis influenced cell apoptosis. An increased TS protein expression seen in many cancers, serving as aggressive phenotype. Cancers is TS disease, still without a cure, taking away the lives of at least 8 million people every year irrespective of nationality, remained a burden that let world health organization to request for urgent treatment. The trouble encompassing cancer in general and that which generated from TS is the principal base of this review. Although, it has been used in other disease and have not yield total cure in cancer yet, TSI potentially utilized to offset the problem responsible for low circulated thymidine in human plasma, is useful to manage cancer disease. Members of TSI bears five or six cycle ring in their structure. The pterine and pyrimidine are compounds which are incorporation with another compound by utilizing the technique of nucleophilic displacement, multicomponent condensation and ultimate combination principle, molecular hybridization strategy and the structural activity relationship mechanism to develop new small TS inhibitors. Through the available English articles about TS related cancer and TSI in ScienceDirect database are utilized here. The search was made by key in the keywards singularly and in pairs to present this comprehensive knowledge about TSI inhibitors, and evaluate TS enzyme mechanisms which contributes to cancer and how the TSI can circumvent cancer disease. Conclusively, the series of TSI utilized the TS protein enzyme to ombat TS human cancer, are also in use as a fundamental molecular template for new drug. TS activity can produce the novel thymidylate. This work will be of prudent to researchers in drug design and synthesis and as well to hospital clinician to support the judgement inside cancer disease treatment.
Thymidylate synthase; Thymidylate synthase inhibitors; Cancer; Folate; Deoxyuridine; Deoxythymidylate triphosphate
Thymidylate synthase (TS); drug target suitable for TS inhibitors, play a necessary role in DNA and deoxythymidine monophosphate (dTMP) synthesis, is a protein that contained genes of the nucleotide polymorphism which influenced cancer development, and commonly referred to as bi-substrate nucleotide enzyme (Khairullina et al, 2018; Karunaratne et al, 2017; Feng et al, 2019). Researchers derived cancer drug target from detection of gene(s) that changes without modification; epigenetic study, of which most studies indited Histone lysine and histone demethylase in human cancer (Yang et al, 2019; Ma QS et al, 2019). The study about cancer disease and its regulators; especially inhibitors began for ages, however, the aggressive cell disease is still taking away the lives of many people making itself the topmost world health organization public health disease that is responsible for 8 million and more deaths until 2025 (Ferlay et al, 2012; Abramsa et al, 2018; Roca et al, 2019). The initial aim of the review. Records about cancer prognostic improvement is noted in recent years. Therapies are based on molecular tumour features (Favaretto et al, 2009; Agustonia et al, 2019). Regimen for this terrible epidemic is limited to surgery, irradiation, radiotherapy, and chemotherapy, but, are followed with obstacles known as Resistance and delayed in disease diagnosis. Chemotherapeutics are more tolerated, and classified into the first, second and third generation, and the technique involved blockade of cell proliferation and reduction of resistant cell growth (Agustonia et al, 2019; Sacchetti et al, 2015; Infantino et al, 2019; Obara et al, 2005; Giudice et al, 2007; Pathania et al, 2018). Cancers are influenced by mutations and elevated TS protein (Rahman et al, 2004). The TS cultivates cancer, is another base for the review, through the ability to mingle in cell division and to drive in uracil into DNA which causes the breakage of DNA strand(s) Somehow TS impair facilitates the imbalance between deoxyuridine triphosphate (dUTP) and deoxythymidine triphosphate (dTTP), as well as cell arrest and apoptosis (Raghavendra et al, 2018; Chon et al, 2017).
Three forms of this rate-limiting TS protein evident as homodimeric ThyA, homotetrameric ThyX and the thymidylate synthase X which is present in bacterial only, but not the later involved in cancer formation (Önen et al, 2008; Jackman et al, 2002). Their genes fulfill the replication process; controlled transcription with factor 28-bp tandem repeats in promoter region and then can only bind to its own mRNA for translation. TS structure has an amino group at position 106 necessary for N-ethyl-N-nitrosourea mutagenesis. Recently, TS expression is higher in individuals with the 3R allele compared to the 2R allele and this has an impact on cancer treatment and development. TS mRNA levels increased to as much as 20-fold in S-phase DNA synthesis drove by the E2F transcription factor which is control by the expression of other proteins required for cell cycle progression, DNA damage response, and stress response. Thymidylate synthase X is the target for antibacterial agents (Chon et al, 2017; Onen et al, 2008; Jackman et al, 2002; Ferrari et al, 2018; Kang et al, 2018). TS mostly work together with other nucleotide molecule mainly to support the production of basic thymidylate. Therein the replenished and imbalanced in phosphate pool highly responsible for the increased rates of uracil uptake into the DNA. The enzyme; DNA polymerases can incorporate monophosphate (dTMP) or triphosphate (dTTP) compound when there is an “A” base on the template strand, and that causes increase dTMP and limiting dTTP in DNA whiles the uracil in DNA repair responsible for enzymatic breakage in the DNA single or double -strand (Chon et al, 2017; Onen et al, 2008). Jarmuła A. et al 2017 reveal the capability of TS to form complexes using three crystal nematode thymidylate synthase (TS) as a Caenorhabditis elegans enzyme without ligands but can form a ternary complex with dUMP and Raltitrexed, also a binary complex of Trichinella spiralis (Ts) enzyme with dUMP (Jarmułaa et al, 2017).
This study was built crave way for more research to design treatment for TS related cancer. We highligh the involvement mechanism of TS protein in cancer, and its usefulness in the production of novel thymidylate which is influential in human body functionalities like metabolism.
TS – Folate enzyme influenced thymidylate production; mechanism
Designed TS cycle below, by Karunaratne K et al., 2017, encompassed with diverted routes which are categorized as flavin and non-flavin. An observed essentially transfer of methylene catalysed the 2-deoxyuridine 5 monophosphates (dUMP) to produce deoxythymidine monophosphate (dTMP) which then, dTMP continues with phosphorylation that ends up with the production of deoxythymidine-triphosphate (dTTP); a DNA precursor. Folate molecule facilitates the transfer process; at carbon 5 uracil moieties. The synthesis started in the nucleus, phosphorylation in a human cell is similar to that of recombinant protein in bacterial cells (Rahman et al, 2004; Raghavendra et al, 2018; Chon et al, 2017; Önen et al, 2008; Jackman et al, 2002; Ferrari et al, 2018; Kang et al, 2018; Jarmułaa et al, 2017; Wang et al, 2018; Frączyk et al, 2015). TS and another small proteins therein work to modify, stabilize and enhance the activity in the cycle. Include in, enzymes like the tetrahydrofolate (THF), 5, 10-methylenetetrahydrofolate reductase, serine hydroxyl methyltransferase and others compete with TS for essential carbon transfer during the process of thymidylate synthesis. The transferred carbon arose from Formate, serine, glycine, sarcosine, dimethylglycine or histidine molecule. Mostly, they are carried by the enzyme tetrahydrofolate (THF). As noticed even in ATP-generating reaction. Attached to the TS cycle is the folates cycle. Here, tetrahydrofolate can oxidized to dihydrofolate is a reverse animation involving dihydrofolate reductase which catalyzed dihydrofolate to tetrahydrofolate in the nicotinamide adenine dinucleotide phosphate (NADPH) -dependent reduction phase (Chon et al, 2017; Önen et al, 2008; Jackman et al, 2002; Ferrari et al, 2018). Without the involvement of the TS enzyme, thymidylate is generated from scavenging exogenous thymidine compounds in the presence of tyrosine kinase. TS cannot directly synthesize thymidylate from RNA, however, it is recognised that RNA can be attached to protein TS and enabling them to regulates their mRNA and other cellular mRNAs. Research has ascertained the increase in TS protein can lead to stabilization of the protein due to geometric changes in the ternary complex (Karunaratne et al, 2017; Liet al, 2018; Ackland et al, 2006). The activity encircle enzymes involved in the production of thymidylate responsible for the replenishing of THF molecule. In the Flavin dependent cycle, the FDTS catalyzed dTMP formation as flavin is reduced by NADPH. Whiles in the oxidative half- reaction, the flavin is oxidized to form dTMP (Karunaratne et al, 2017; El Asrar et al, 2017).
Analog molecules to TS
TS analog molecule facilitates the processes of new TS inhibitor, are produced by modifying the nucleotides phosphate, sugar, and nucleobase moieties in TS molecule. Nucleotides make up the DNA and RNA is significant in cell expression, regulation, and generation of gene products, are building blocks of life. Efficiently the TS analog design to stop the TS related diseases. An example is exocyclic olefin isomer of thymidylate; intermediate for the thymidylate biosynthesis in pathogenic organisms; synthesis is from preserving the glycosidic bond of thymidine and manipulating the base to generate an exocyclic methylene moiety (Mondal et al, 2018). Folate analogs are necessary for the production cycle of antifolates, potent in blocking the enzymatic activity leading to disease that encircles TS (Chon et al, 2017). The 50-monophosphates of 5-substituted 20-deoxyuridine analog can suppress tuberculosis thymidylate synthases; classical (ThyA) and flavin-dependent thymidylate synthase (ThyX) (Alexandrova et al, 2015). Xue H et al, 2019 brought chalcone analogue of the 4-oxoquinazolin-2-yl group which inhibit proliferation and its motif contributes to essential biological processes. The quinazoline ring makes this analogue essential (Han et al, 2019).
Itself; TS protein together with the analog involved to predict disease state, select treatment strategies and prognosis outcome of diseases. In the cancerous cell, there is a rise in thymidylate and DNA synthesis process that increased TS activity (Figure 1) (Liet al, 2018; Ackland et al, 2006).
Thymidylate synthase inhibitors (TSI)
The existing molecules with potential to targets the TS are fragmented TS regulators, have inhibitory antitumor activity, and still serving as a template in new drug discovery. They are designed through structural activity relation method involved in also nucleophilic displacement, multicomponent condensation; ultimate combination principle, Molecular hybridization strategy, etc. (Khairullina et al, 2018; Mondal et al, 2018; Alexandrova et al, 2015; Han et al, 2019; Kumar et al, 2014; Zaware et al, 2017; McClellan et al, 2011). Their activity was confirmed by in-vivo and vitro tests, and were found to bind to the active site of substrates or the non-substrate analog. They could be direct and indirect acting regulators, primarily, are to offset the problem with low circulated thymidine in human plasma, loss of thymine in cancerous cells and of abnormal DNA synthesis that is responsible for Cell apoptosis (Ackland et al, 2006; El Asrar et al, 2017; Mondal et al, 2018; Alexandrova et al, 2015; Han et al, 2019; Kumar et al, 2014; Zaware et al, 2017 McClellan et al, 2011; Catalano et al, 2016; Ittarat et al, 2018). However, it worth wise that molecules with dual-Multi targeting for cancer protein are better than single compound that target single protein as the single compound is prompt to suffer the crisis of drugs resistant and overexpression (Khairullina et al, 2018; Khairullina et al, 2018, Kang et al, 2018; Liet al, 2018; Toivonen et al, 2018). Below are some useful TSI in existence.
Oxadiazoles
Oxadiazoles molecule bears an oxadiazole moiety as seen in the Ethyl 5-trichloromethyl-1, 2, 4-oxadiazole-3- carboxylate, 5-Amino-1, 2, 4-oxadiazoles, is synthesized from different techniques of which one involved Beckmann rearrangement reaction, the reaction of cyanamides and have to deal with the N-hydroxyamidine. This molecule has established in pharmacophore as integrated biologically relevant molecule with ability to convert compounds in other to improve their biological properties (Jakopin et al, 2018). Its potential can block the activity of thymidylate synthase (TS), telomerase, histone deacetylase, methionine aminopeptidase, glycogen synthase kinase-3, epidermal growth factor, vascular endothelial growth factor and focal adhesion kinase. It blockades activities vary, depend on the substituents that are attached at the benzene ring which is joined to 1, 3, 4-oxadiazole, and also can increase the incorporation of electron withdrawing groups into the phenyl ring (Alikhani et al, 2018). The compound containing oxadiazole can induce apoptosis in HepG2 cancer cells (Yang et al, 2019). In colourimetric MTT test with cancer cell lines 4T1 – mammary carcinoma, CT26.WT – colon cancer cells and one non-tumour cell line and BHK- 21 –baby hamster kidney effectiveness of this molecule had been performed however, from strong to moderate cytotoxic activities was observed against K-562, KG-1a, and Jurkat leukaemia cell lines in MTT assays (Fathi et al, 2019).
Pyrimidines
The pyridine molecules of the type thiophenes and thienopyrimidines are known worldwide and bear in thiophene ring. Morpholine and piperazine moieties are added to the structure of thienopyrimidines with a thieno [2,3-d] pyrimidine-4(3H)-one ring, provide them with the potential to block thymidylate synthase and protein kinase enzymes, and also, to possess good DNA binding affinity and inhibitory activity which is necessary in disease management including viral infection, microbial, inflammatory and cancer diseases. The thienopyrimidine derivatives are effective against five cell lines namely; hepatocellular carcinoma (liver) HepG-2, epidermoid carcinoma (larynx) Hep-2, mammary gland (breast) MCF-7, human prostate cancer PC-3 epithelioid cervix carcinoma HeLa and receptor tyrosine kinases EGFR, ErbB-2, ErbB-3, and ErbB-4 (Fouad et al, 2018; Rheault et al, 2009). Suitably, it can inhibit the activity of notum pectin acetylesterase and platelet aggregation. Prospectively, the moiety of this product can replace pyrrolopyrimidine residue to form a family of potent and selective inhibitors for Aurora kinases; serine-threonine kinases which are key regulators for mitotic progression (Tarver et al, 2016; Kortum et al, 2009). Thiophene alone has eminent efficacy against bacterial Escherichia coli ATCC 12435, Bacillus cereus UW 85 and Staphylococcus aureus, Candida albicans and Aspergillus fumigatus 293 than ampicillin (McClellan et al, 2011). Recently, 6-substituted pyrrolo [2,3-d] pyrimidines discovered and have the capacity to target both thymidylate and purine nucleotide biosynthesis. Potentially, pyrimidines antiproliferative is potent for tumour cell lines such as the KB, SW620 and MCF7, folate- dependent enzymes thymidylate synthase (TS), glycinamide ribonucleotide formyltransferase (GARFTase) and 5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase (AICARFTase) resulting in distinct early apoptosis and cell cycle arrest at S-phase and ultimate cell death (Xing et al, 2017). Varano F et al, 2018 synthesised thiazolo [5,4-d] pyrimidine molecule which has effective affinity and potency for human adenosine receptors expressed in CHO cells (Varano et al, 2018).
Sulfonamides derivatives
Sulfonamide bears in structure a piperazine and piperidine sulfonyl. Gamage S. A. et al 2017 involved chloro-triazines in the production of sulfonamides that condensed with Boc which protects the piperazine derivatives and the triazines. Removal of the Boc group by treatment with TFA in CH2Cl2, followed by the reaction of amines with the appropriate sulfonyl or sulfamyl chlorides (Gamage et al, 2017). Replacement of the piperazine ring with weak base morpholine core resulted in a reduction in inhibitory activity which is restored by shortening the linkage from methyleneoxy to oxygen and leads to the production of benzenesulfonamide inhibitors (Wu et al, 2018). The molecule has a blockade effect on dihydrofolate reductase enzyme and many human cancer cell lines. It has been used as antiproliferative molecule against leukaemia (cell lines K-562 and MOLT-4), non-small cell lung cancer (cell line NCI- H522), colon cancer (cell lines NT29 and SW-620), melanoma (cell lines MALME-3M and UACC-257), ovarian cancer (cell lines IGROV1 and OVCAR-3), renal cancer (cell lines ACHN and UO-31), breast cancer (cell line T-47D), to suppress tumor invasion, and to reversal the acidification effect of tumor (Kachaeva et al, 2018; Okolotowicz et al, 2018). The molecule is useful in other disease includes viral and bacterial infection like HIV-1. It anticancer activity at the cell cycle G1 phase is the interruption of microtubules and blockade of angiogenesis. The active form with aromatase an H-bond and pep-stacking without chelation of the heme iron. Note wisely, the H-bonding is a water-mediate network that facilitates the interaction between the aromatase and the keto oxygen of KSHV thymidylate synthase complex (Fahim et al, 2019; Matteo et al, 2016). Some sulphonamide like the 4- Chloro-3-nitrobenzenesulfonamide in room temperature can react with a range of biselectrophilic phenols through NH-acidic functionality in the ortho-position, and the product is effective against human carbonic anhydrases which are primary regulators of pH within and outside the cell and catalyze the reversible hydration of carbon dioxide to produce bicarbonate anion and a proton (Sapegin et al, 2018). Potently the this ompound capable to replace morpholine groups attached to phosphatidylinositol 3-kinase (PI3K), inhibitor 2- (Difluoromethyl)-1-[4,6–di (4-morpholinyl)-1,3,5–triazin-2-yl] -1 H-benzimidazole (ZSTK474) to produce a new class of active and potent PI3Ka inhibitors. The derivatives 1, 3- diaryltriazenesubstituted sulfonamides discovered to inhibit four selected human carbonic anhydrase isoforms (hCA I, hCA II, hCA VII and hCA IX) but as well useful in the treatment of numerous diseases such as glaucoma, epilepsy, retinitis pigmentosa, cancer, obesity, etc (Lolak et al, 2018).
5- Fluorouracil (5-FU)
5-FU have in its fluoropyrimidines ring, enhanced peritoneal absorption of hyperthermicintraperitoneal chemotherapy oxaliplatin (Badrudin et al, 2018; Zou et al, 2017) is a potential template as well for new drug especially for colorectal cancer (Khan et al, 2018; Kelly et al, 2013), and rectal cancer (Yoney et al, 2008). Presently, it is in use as an effective treatment for renal cancer (Zheng et al, 2018). 5-FU microparticles are prepared by supercritical antisolvent (SAS) precipitation using dimethyl sulfoxide solvent (Cuadra et al, 2019). In the presence of natural product such as phytosterols, no toxicity was noticed (Álvarez-Salaa et al, 2018). 5 FU mediates cell death process, binding potency to 5, 10-methylenetetrahydrofolate and can form a ternary stable complex, and produced metabolite known as 5- fluorodeoxyuridine (5-FdUMP) which is generated by thymidine phosphorylate protein. In thymidylate synthesis, 5 FU cause an irreversible inhibition to TS protein. The metabolite alone or in combination is effective against some human tumour cells, but, low therapeutic stability and efficacy limit their use (Khairullina et al, 2018; Ackland et al, 2006). Mostly it is used in combination with leucovorin or capecitabine (Deboever et al, 2013) and its injections preferable for cosmetically sensitive skin areas than that of triamcinolone (TAC) (Hietanen et al, 2019). Patient with progressive 5-FU resistance after initial responding to 5-FU treatment can respond to Rosmarinic acid which enhances chemosensitivity of resistant gastric carcinoma SGC7901 cells to 5-Fu by downregulating miR-6785-5p and miR-642a-3p and increasing FOXO4 expression (Yua et al, 2019). Although it accompanies with toxicity, however, the molecule oridonin enhances the cytotoxicity of 5-FU in renal cancer cells through inducing necroptosis (Zheng et al, 2018). Together; WPMP-2 conjugate and-fluorouracil-1-acetic acid (5-FUAC reduce immunosuppression of 5-fluorouracil (5-Fu) (Xu et al, 2017). The immune-compromised patients are at higher risk of developing toxicity. Some patients report with -FU toxicity and were possibly recover after treatment, even patients with HIV/AIDs (Santos et al, 2017). It was implanted in the peritoneal cavity to impede colon cancer growth. Through the local sustained release, it enables effective antitumor activity and decreased side effect (Li et al, 2018) As well, it serving effective for keloid and hypertrophic scar and presents a lower rate of recurrence (Khalid et al, 2018). In molecular dynamics (MD) simulations, cholesterol enhances the effect on the interaction between the hydrophilic 5-FU and fully hydrated 1, 2-dimyristoyl-sn-glycerol 3-phosphocholine (DMPC) bilayer. The drug molecules 5-FU are accumulated in the vicinity of the polar head group of lipid bilayers and with increasing cholesterol concentration in the bilayer, the free energy barrier for translocation of 5-FU across the bilayer increases because of the condensing effect of the cholesterol on the bilayer (Khajeh et al, 2014).
Folic acid
Folic acid bears in its structure a pteridine, possess molecular recognition for folate receptors, can regulate DNA synthesis and activity of tumor cells, is of high cytotoxic effect for some cell lines like keratinocytes and fibroblasts (Borah et al, 2018; Pagano et al, 2019). It capable to improve the bioavailability of linkage complexes which are necessary for photodynamic therapy activity on MCF-7 cells over amine functionalized magnetic nanoparticles carriers (Matlou et al, 2018), provide antioxidant effects and prevent neural tube defects in woman for their offspring when started within 5-6 months before conception with a dosage of 4 g (Toivonen et al, 2018; Gool et al, 2018), and mitigate the risk of hypertensive disorders (De Ocampo et al, 2018). Conjugate contained the molecule can enhance efficacy and responsible for the reduction in dosage for some chemotherapeutic drugs (Martino et al, 2018; Bandara et al, 2018). Possible to leads to homocysteine accumulation and epigenetic dysregulation associated with one-carbon metabolism and provide the protection of undifferentiated cells from oxidative damage by targeting mitochondrial activation, decreased amount in mitochondrial reactive oxygen species, and accompanied with PGC-1a upregulation, DRP1 downregulation, mitochondrial elongation, and increased ATP production (Zhang et al, 2019). In structural analysis the tRNA binds folic acid-PAMAM through H-bonding which is hydrophobic and van der Waals contacts, however, the PAMAM size increases the binding efficacy and the stability of tRNA conjugates. Folic acid-PAMAM nanoparticles can transport tRNA in vitro (Chanphai et al, 2018). Folic acid (FA)-induced acute kidney injury. The renal function depends on mitochondrial homeostasis wherein mitochondrial alterations are known as developmental contributor. This molecule is used in model for studies about renal damage and its progression to chronic state. It causes mitochondrial and renal dysfunction which is possible to be protected by N-acetyl-cysteine (NAC) through preserving the S-glutathionylation process and GSH levels in mitochondria (Aparicio-Trejoa et al, 2019). In combination, such as that with zinc, folic acid control depression through increases the levels of 5-HT, DA and NE in the brain, and up-regulate Trk B and NMDA (Dou et al, 2018). Essentially, the Folic acid supplement reduced colorectal cancer risk. However, the bioavailability and metabolism parameters are necessary during assessment is a main reasons for the progress about the molecule (Moazzen et al, 2018). In hepatocellular carcinoma developed by non-alcoholic fatty liver disease, increase risk factor was from Low serum folic acid level in the Chinese population. On the other hand, addition of the serum folic acid levels to the current existing non-alcoholic fatty liver disease result in significant improvement of the NAFLD patients (Xia et al, 2018).
Pemetrexed
Discovery of this molecule was in the late 1990s, purposely was to block the enzymes in folate cycle such as thymidylate synthase, dihydrofolate reductase, and glycinamide ribonucleotide formyltransferase (Favaretto et al, 2009; Molina et al, 2003). The antifolates including pemetrexed are used singularly or in combination with other chemotherapy in management of cancers including breast, mesothelioma, lung, colon, pancreatic, gastric, bladder, head and neck, cervical and urothelial and kidney cancers with acceptable toxicities (Zhou et al, 2015; Kulkarni et al, 2016; Sternberg et al, 2003). Pemetrexed is third-generation cytotoxic drug and antimetabolites work together with non-coding RNAs; small microRNAs and long non-coding RNAs (Petrelli et al, 2018; Biersack et al, 2018; Biersack et al, 2018). This chemical is approved by Food and Drug Association for initial treatment of metastatic non-squamous NSCLC to use together with cisplatin. Potentially, it can block the synthesis of purine and pyrimidine, as well as nucleotides that is required for the formation of DNA and RNA. Two prognostic biomarkers in NSCLC which plays key role in DNA repair mechanisms; uracil DNA glycosylase (UDG) and BRCA1, significantly (Favaretto et al, 2009) shorter Progression-free and overall survival (Sadraei et al, 2018). It combination with platinum is first-line therapy for mesothelioma; tumor of the pleura consisted of mutation of the gene CDK2NA and cyclin-dependent kinase control with subsequent loss of cellular cycle regulation as well as exposure to asbestos (Petrelli et al, 2018; Biersack et al, 2018; Biersack et al, 2018; Sadraei et al, 2018; Bronte et al, 2016; Scherpereel et al, 2018) and once served as alternative to the conventional NSCLC as second line treatment (Favaretto et al, 2009) but currently is the first-line agent for advanced nonsquamous NSCLC. Report of pemetrexed broad spectrum activity in CRC and pancreatic cancer treated together with gemcitabine, irinotecan, and oxaliplatin have also proved feasible (Simon et al, 2013; Patel et al, 2018). Pemetrexed and epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs) combination is associated with higher incidence of grade 3/4 treatment-related adverse events than the single-agent regimens (Yang et al, 2017). Analysis view came out that pemetrexed similar to methotrexate in inhibiting the dihydrofolate reductase (DHFR) and that analogous to 5-FU and raltitrexed in inhibiting TS. In contrast to these other agents, pemetrexed has ability to target several enzymes and capable to results in decreased acquired resistance (Molina et al, 2003).
Raltitrexed
Raltitrexed; a quinazoline folate analog serving mostly as monotherapy and first-line treatment for colorectal cancer, and other cancers such as the cancer of the head and neck, mesothelioma, pancreatic and gastrointestinal cancer. It is direct and specific thymidylate synthase inhibitor which enters the cells rapidly through reduced-folate carrier, and can blocks the production of thymidine monophosphate from deoxyuridine monophosphate, leads to DNA fragmentation and cell death. Recently, drug registration report showed this molecule is alternative to 5-FU especially for people with fluoropyrimidines-related cardiac toxicities together with those connected to drop down of creatinine clearance, sand possesses prolonged TS inhibitory activity due to the rapid formation of polyglutamates and subsequent retention within cells. Also, Raltitrexed is radiosensitiser that is applicable in rectal cancer (Khan et al, 2018; Kelly et al, 2013; Liu et al, 2014; Hu et al, 2007; Cunningham et al, 2002; Popovet al, 2008; Planting et al, 2005). The combination of raltitrexed with platinum-based agents and/oranthracyclines is effective for advanced cancer condition (Armandet al, 1999). and with oxaliplatin is a promising combine therapy for mesothelioma cancer (Fizazi et al, 2000; Porta et al, 2005) In neoadjuvant chemoradiation condition it promote high pathologic tumor down staging and sphincter-saving procedure (Gambacorta et al, 2004). Naphthahmides, Methotrexate, ZD9331, Quinazolin deravatives, Pralatrexate, and N-phenyl-(2, 4-dihydroxypyrimidine-5-sulfonamido) benzoyl hydrazide.
The molecule of Naphthahmides bears in nitro or substituted-amino groups to the naphthalic ring, two phenolic hydroxyl groups and lactonic carbonyl. The modification in lactonic moiety with imidic derivatives is likely responsible for increased stability at low micromolar concentrations for inhibitors of Lactobacillus casel TS (Costil et al, 1996).
Methotrexate can block dihydrofolate reductase (DHFR) and the biosynthesis of thymidylate in tumour cells, are applicable in the management of autoimmune disorders disease, cancer, cytostatic and immune disease. Its binding is irreversible. Note wisely, DHFR inhibitors can block purine biosynthesis which leads to resistance cells (Karunaratne et al, 2017; Ackland et al, 2006).
ZD9331 is folate-based potent specific TS inhibitor, useful for ovarian, and colon cancers. Its anticancer activity is independent of folylpolyglutamyl synthetase (FPGS) activity. It can be transported into cells through the reduced-folate carrier (RFC), and it is not metabolized by folylpolyglutamyl synthetase (FPGS) but subjected to slow terminal phase plasma clearance in humans (Jackman et al, 2002).
The 2-amino-quinazoline-4-one and 2-methyl-quinazoline-4-one bears in their structure a quinazoline ring, can block DHFR and purine biosynthesis, have broader spectrum activity and higher therapeutic efficacy. Some new members are undergoing clinical trials as potential antitumor drugs. However, high hepatic and renal toxicity couple with poor solubility are responsible for their limitation Table 1, (Karunaratne et al, 2017).
| Molecule/antimetabolite | Target and structure | Reference |
|---|---|---|
| Pemetrexed | 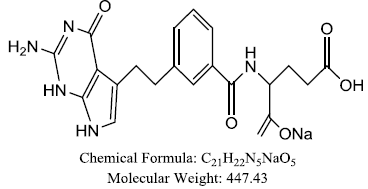 |
Li X, et al. |
| Methotrexate | 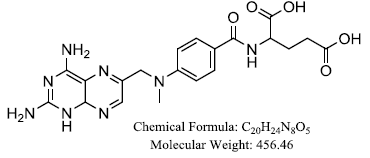 |
Li X, et al. |
| N-phenyl-(2,4- dihydroxypyrimidine-5- sulfonamido)benzoyl hydrazide | 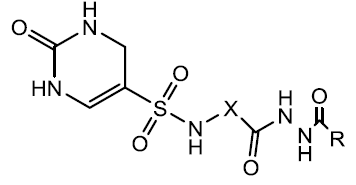 |
Li X, et al. |
| Raltitrexed | 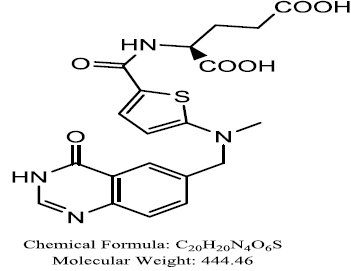 |
Li X, et al. |
| 5-(arylthio)-9H- pyrimido[4,5-b]indole-2,4- diamines | 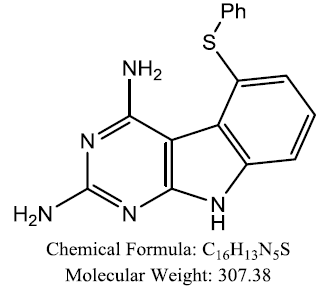 |
Zaware N, et al. |
| Capecitabine | 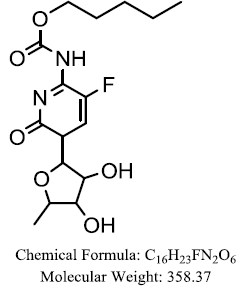 |
Zaware N, et al. |
| Quinazolin-4-one derivatives | 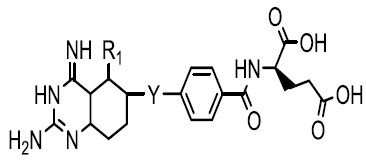 |
Khairullina VR, et al. |
| quinazolin-4-imine derivatives | 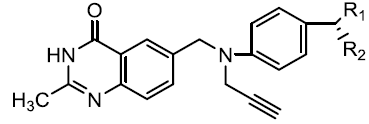 |
Khairullina VR, et al. |
| Folic acid | 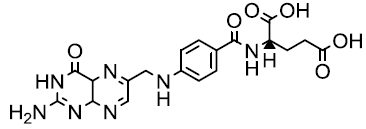 |
Khairullina VR, et al. |
| 5,10- methylenetetrahydrofolate |
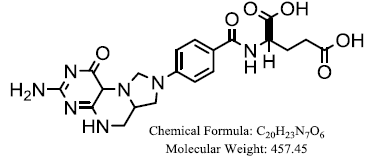 |
Khairullina, VR, et al. |
| CB 3717 | 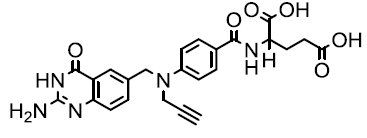 |
Khairullina VR, et al. |
| ZD 9331, | 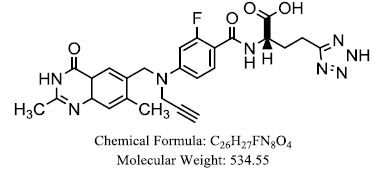 |
Veronika R, Khairullina, et al. |
| 5- Fluorouracil (5-FU) Direct anti TS | 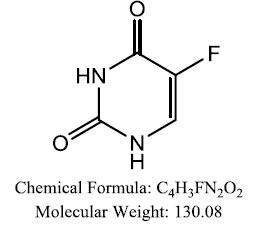 |
Khairullina VR, et al. |
| 5- fluorodeoxyuridine (5- FdUMP) | 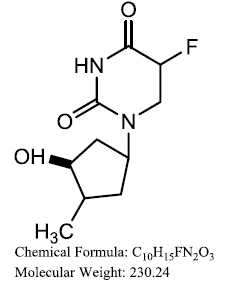 |
Khairullina VR, et al. |
| BW 1843U89 | 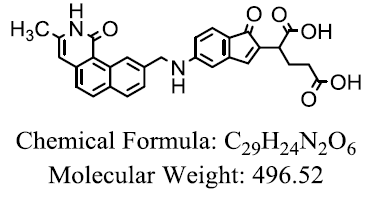 |
Khairullina VR, et al. |
| AG 337 | 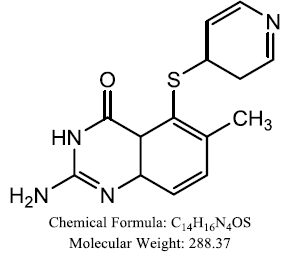 |
Khairullina VR, et al. |
| Tomudex | 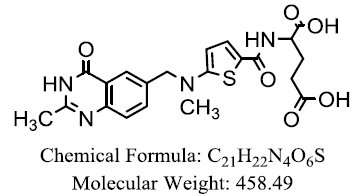 |
Khairullina VR, et al. |
| Tegafur | 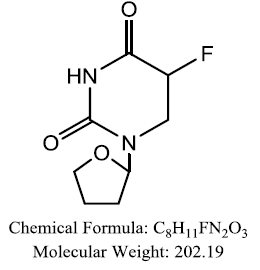 |
Khairullina VR, et al. |
| Leukovorin | 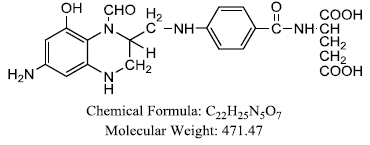 |
Ackland SP, et al. |
| Thiophene | 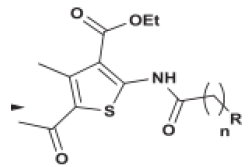 |
Fouad MM, et al. |
| Thienopyrimidine | 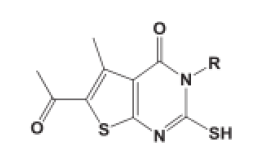 |
Fouad MM, et al. |
| Benzenesulfonamide derivatives | 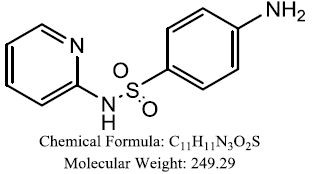 |
Fahim AM, et al. |
| Naphthalimides derivaties | 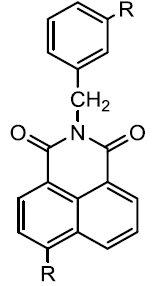 |
Costil M, et al. |
| 6-substituted pyrrolo[2,3-d] pyrimidines | 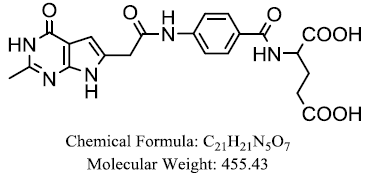 |
Ittarat W, et al. |
| Pralatrexate | 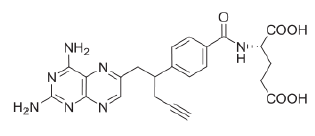 |
Xing, Ruijuan, et al. |
Pralatrexate was approved in 2009 as chemotherapy for relapsed or refractory peripheral T-cell lymphoma. It contains 2, 4- diaminopyrimidine moiety for binding to the active sites of DHFR (Varano et al, 2018).
N-phenyl-(2, 4-dihydroxypyrimidine-5-sulfonamido) benzoyl hydrazide is newly synthesized TS nucleotide metabolism blocker synthesis in our laboratory, can induce apoptosis, tumour cell proliferation by activating caspase-3, increasing expression of cleaved caspase-3 and reducing the ratio of Bcl-2/Bax (Li et al, 2018).
Elsevier Science direct libraries were utilized, and searched was done in English by key in the keywords; Thymidylate Synthase, Thymidylate inhibitors/blockers, Thymidylate Synthase related cancers, Oxadiazoles, Pralatrexate, pyrimidines, Raltitrexed, Pemetrexed, 5- Fluorouracil (5-FU), Naphthahmides, Sulfonamides, to extract more than 100s of articles with useful related information.
TS protein is cancer biomarker that limited researcher have recorded and urged for more research to be done. The worldwide request for cancer effective inhibitors has warranted much institution to go in search of new potent TS inhibitors that will combat cancer disease of all types and related problem. TS is found in many cancer. Upon this we developed our aim and objective for this review and ransack the Science Direct database. Chemotherapy is the most acceptable and frequently used techniques in cancer management. Cancer is serious cell disease with over-warmly death amount and still taking away the lives of people irrespective of nationality. The failure of existing medication resulted from the development of resistant cancer cell; barrier to drug success which have increased also the burden of the disease. TS synthesized end product; thymidylate is primary factor for cancer. The process encircle the production of thymidylate as depicted in thymidylate synthase cycle above contained pathways and check points that have small nucleotides protease synthase which contribute in cell metabolic processes. The formation of cancer is reported to involved metabolic proteins including DNA and RNA molecules, unresolved gene changes and cell apoptosis mechanism. TS cultivate cancer cells through it involving roles in cell division, driving uracil into DNA, and facilitation of the imbalance between deoxythymidine triphosphate and thymidine triphosphate.
The TS cancer inhibitors block the production of thymidylate, and also those contributing proteins that TS utilized during the production of cancer factors. Advances in drug discovery; previous TS inhibitors are useful template for new effective medication. The combinations theory and structural activity relationship are utilized in drug modification event is a procedure necessary for more compound production which is the hope for patients suffering the disease.
Our study provides evidence, together with simplified and compressed idea about TS protein and TS inhibitors in cancers disease and compile TS inhibitors in a table. It’s of helpful to the drug design scientist and cancer disease clinician in making drug decision.
The authors declared that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by the National Natural Science Foundation of China (Grant No. 81573687, 81274182) and the project of Liaoning Distinguished Professor.